Study Summary
Why are we doing this trial?
Cancer of the sinuses (the air spaces in our face around the nose) is a rare and aggressive disease, which can be extensive by the time something is noticed to be wrong.
The standard of care treatment includes;
- Surgery
- Chemotherapy
- Radiotherapy
Radiotherapy is an important part of the treatment and can be used to treat cancer after surgery but also used where surgery has not been done.
Intensity-modulated radiotherapy (IMRT) is the current standard treatment. It uses high energy photons (x-rays) that are shaped to fit closely to the area of the cancer. This means that the cancer receives high doses of radiotherapy, but the normal healthy cells get less which should cause less side effects.
The use of radiotherapy in the head and neck is complicated because of the closeness to critical areas, (e.g. the eyes and brain) which could be damaged by radiotherapy. This may sometimes cause serious long-term side effects (e.g. loss of vision, problems with thinking, memory, or epilepsy).
Proton beam therapy (PBT) is a newer form of therapy which may further improve the delivery of radiation. It uses protons (rather than photons) which are very small particles. They treat cancer by producing a sudden burst of energy when they stop. During PBT the protons are made to stop inside the cancer which can destroy it. This can reduce the damage caused to the healthy cells.
This trial will compare PBT with IMRT in patients with cancer of the sinuses, aiming to discover which treatment provides better cure outcomes, has fewer side effects and results in better quality of life for patients.
What happens on the trial?
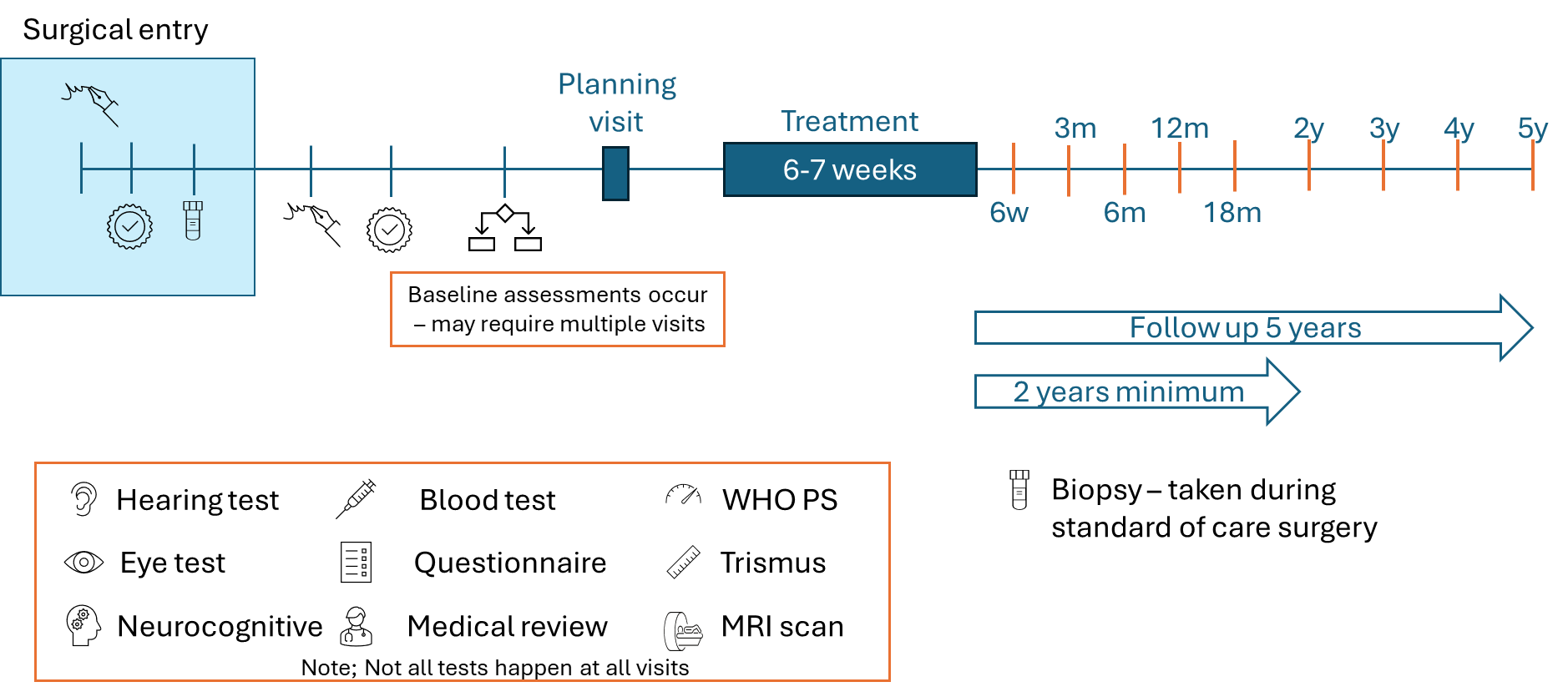
The study wants to recruit 276 patients from across the UK. The image below gives an overview of the PROTIS trial.
Consent
Each participant will confirm in writing that they are happy to take part in the trial following a conversation with their clinical care team.
If a participant is undergoing surgery as part of their care then they can provide a biopsy taken at this time.
Eligibility
Doctors will check whether the trial is suitable for each person based on clinical criteria.
Randomisation
Each participant has a 50:50 chance of receiving IMRT or PBT. A computer assigns treatment; neither participants nor clinicians choose.
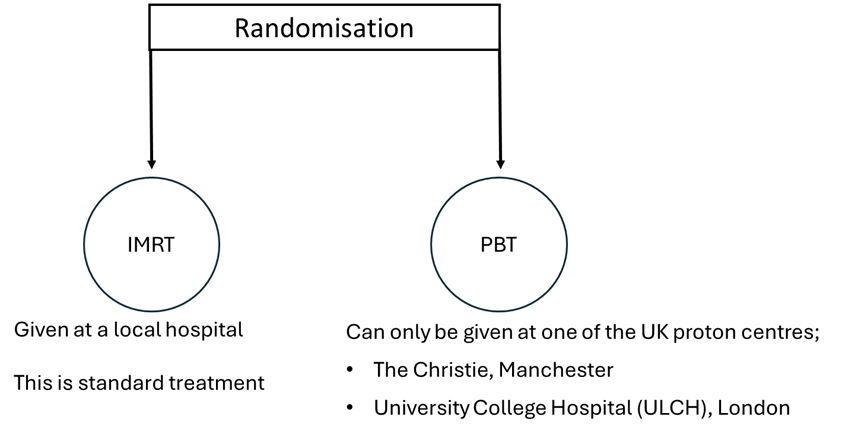
Treatment
Delivery of treatment (IMRT or PBT) will happen over 6-7 weeks , with one “fraction” given once per day, Monday to Friday.
If PBT is the treatment, participants can stay at accommodation provided by the hospital in either Manchester or London (see the Proton Centres links). A partner or carer can stay there too. There is money available via the PROTIS trial for travel to the proton beam centres, which participants can claim back.
Assessments

During the trial participants will undergo several different assessments (as represented in the orange box in the diagram). The first set of assessments will be done before treatment begins – they are called baseline assessments.
After completing the radiotherapy, participants will attend follow-up clinical appointments at set times for a minimum of 2 years (up to 5 years).
Not all assessments are performed at each visit – a more detailed breakdown is provided to participants who are asked to take part in the trial.
These visits allow us to check whether there have been changes in participant health and the use of questionnaires gives us information about participant quality of life.
Overall aim of the trial
The PROTIS trial will urgently investigate proton beam therapy as a new treatment option for people suffering from sinus cancer; this may have long term impact for our treatment options against this aggressive cancer in the UK and beyond.
PROTIS Recruiting Sites
View our interactive map of recruiting sites.